Overview
Diabetes mellitus (DM) is one of the major public health concerns in Malaysia and closely related to complication of heart disease, stroke, endstage renal failure, blindness and amputation. Mi-Glucosenz is a medical screening device that measures blood glucose levels without the need to prick a finger. Using the concept of non-invasive technique, Mi-Glucosenz offers a pain-free and easy method for a patient to monitor blood glucose levels.
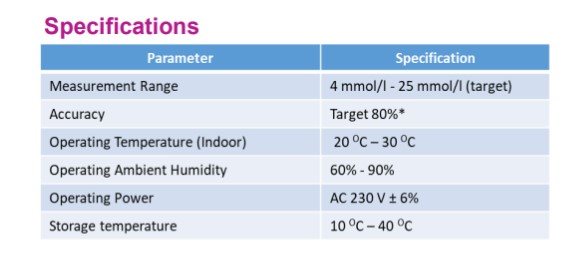
Mi-Glucosenz is a non-invasive blood glucose sensor. Glucosenz uses infrared technology to measure the amount of glucose in blood. The current system is for indoor use. Mi-Glucosenz is used for blood glucose level screening ranging from normal to hyperglycemia. Measurement of glucose is via the human thumb without any needle pricking.
Technology Summary
Mi-Glucosenz
A medical device using non-invasive technique and infrared
technology to measure blood glucose level.
Industries: Enterprise, Government
Features
- Non-invasive
- Pain-free monitoring
- Non-destructive
- IoT-enabled
Technology Benefits
- Painless
- No pricking of skin
- No drawing of blood
Mi-Glucosenz comprises the following features:
Features
Mi-Glucosenz comprises the following features:
- Non-Invasive
No withdrawal of blood sample required. - Pain-Free Monitoring
Pain-free patient monitoring with minimum risk of infection. - Non-Destructive
Evaluate properties of samples without altering or causing any damage. It is reusable. - IoT-Enabled
Ready for future integration into healthcare system.
Technology Benefits
The main impacts of Mi-Glucosenz are:
- Painless
Patient will be pain-free before and after using Mi-Glucosenz. - No pricking of skin
No penetration on any part of human skin to measure glucose level. - No drawing of blood
No blood needed when using Mi-Glucosenz. - Animal Test & Animal Ethical Approval (in collaboration with CUCMS and UPM)
By Jawatankuasa Institut Penggunaan dan Penjagaan Haiwan, UPM (IACUC) - Human Testing (Pre-Clinical, Pilot Run and Clinical
Test)
Human Ethical Approval (in collaboration with CUCMS, Health Medic Sdn Bhd & OSA Technology) Granted approval in June 2015 By Medical Research Ethic Committee (MREC), Malaysia Granted HUKM Ethical approval to conduct related research/human test at HUKM - Accreditation lab testing (SIRIM)
MS IEC 60601-1:2006
CISPR 11 and CISPR 24
CE (Conformité Européene) certification exercise